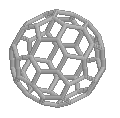
C60 - Buckminsterfullerene

 |
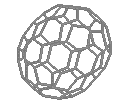 |
| Buckminsterfullerene - C60
(left) and C70 |
The Royal Swedish Academy of Sciences has awarded the 1996 Nobel Prize for Chemistry jointly to:
- Professor Robert F. Curl, Jr., Rice University, Houston, USA
- Professor Sir Harry W. Kroto FRS, University of Sussex, Brighton, UK
- Professor Richard E. Smalley, Rice University, Houston, USA
For their Discovery of Fullerenes
The serendipitous discovery of a third allotropic form in 1985, uncovered a fundamentally different structure of closed carbon cages, which were to become known as fullerenes. This new family of non-planar carbon compounds has generated immense interest within the scientific community in such a short period of time, with thousands of papers published about fullerenes and fullerene-based materials to date.
In the early 1970's, the chemistry of unsaturated carbon configurations was studied by a group at the University of Sussex, led by Harry Kroto and David Walton. They developed methods for synthesising long chain polyynes, whose vibration-rotation dynamics were studied by microwave spectroscopy. They then used these observations for molecular radioastronomy. From 1975-78 they studied the long-chained polyynylcyanides, HC5N, HC7N and HC9N. These molecules were detected in the cloud material of the interstellar medium by radioastronomy. These molecules turned out to be produced by red giant stars.
In the 1980's a technique was developed by Richard Smalley and Bob Curl at Rice University, Texas. They used laser vaporisation of a suitable target to produce clusters of atoms. Kroto realised that by using a graphite target, that the cluster apparatus would be ideal to probe the formation of carbon chains, and so planned a collaboration between his group at Sussex and the one at Rice.
The Sussex/Rice experiment took place in September 1985. The technique probed the carbon plasma produced by the laser vaporisation by time-of-flight mass spectrometry. The experiments confirmed that large carbon chain/clusters were being formed. During the experiments it was noted that the peak for the C60 molecule (and to a lesser extent C70) behaved unusually and formed under all conditions as well as exhibiting great stability.
The experimental evidence, a strong peak at 720 amu (atomic mass units), indicated that a carbon molecule with sixty carbon atoms was forming, but provided little structural information. The research group concluded after reactivity experiments, that the most likely structure was a spheroidal molecule. Kroto mentioned Fuller's geodesic dome structures, which contained pentagons as well as hexagons. The idea was quickly rationalised as the basis of an icosohedral symmetry closed cage structure. The geodesic and electronic bonding factors in the structure accounted for the stability of the molecule, and it was named after Buckminster Fuller.
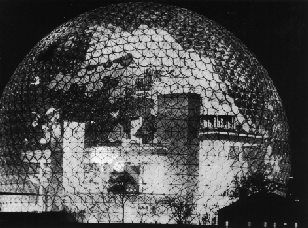 |
| Buckminster Fuller's Dome - Expo '67 Montreal - Courtesy of B. Eggen (Univ. of Sussex) |
Beam-experiments conducted between 1985-90 provided more evidence for the stability of C60 as well as supporting the closed cage structural theory and predicting some of the bulk properties such a molecule would have. Around this time, intense theoretical group theory activity also predicted that C60 should have only four IR
active vibrational bands, on account of its icosohedral symmetry.
In 1989, the Heidelberg/Tuscon group, led by physicists Wolfgang Krätschmer and Donald Huffman had observed unusual IR and UV features in thin carbon films produced by arc-processed
graphite rods. Among other features, the IR spectra showed four discrete bands in close agreement to those proposed for
C60. A paper published by the group in 1990 followed on from their thin film experiments, and detailed the extraction of a benzene soluble material from the arc-processed graphite. This extract was found to crystallise and X-ray analysis consistent with arrays of spherical C60 molecules, approximately 7Å in diameter. The C60 molecule has two bond lengths - the 6:6 ring bonds can be considered "double bonds" and are shorter than
the 6:5 bonds - see below - the "shorter bonds" are highlighted for clarity:
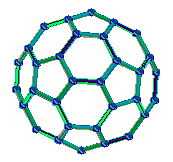
Fullerenes are closed cage structures. Each carbon atom is bonded to three others and is sp2 hybridised. Hexagonal rings are present but pentagonal rings are required for the cage to close.
Mass spectrometry has been widely used to study the fullerenes. There is evidence for species as small as
C20+, as well as stable peaks for the cluster ions C2n+ (where 2n>32). Fullerenes which are stable or abundant enough to exist in macroscopic quantities have
been studied further using a wide range of physical and spectroscopic methods. C60 and C70 have similar properties, with six reversible, one electron reductions to
C606- and C706- having been observed, whereas oxidation is irreversible. The first reduction for both fullerenes is ~1.0 V (Fc/Fc+), indicating they
have electron accepting properties. C76 exhibits both electron donor/acceptor properties.
C60 has a tendency of avoiding having double bonds within the pentagonal rings which makes electron delocalisation poor, and results in the fact that C60 is not "superaromatic". C60
behaves very much like an electron deficient alkene and readily reacts with electron rich species.
 | 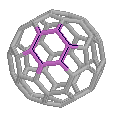 |
A highlight of one of the
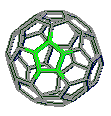
pentagonal rings |
A highlight of one of the
hexagonal rings |
EXERCISE: List at least three important contributions to society provided
by Buckminster Fuller. List three important contributions that fullerenes have
made to science.
|