ALUMINUM
Aluminum is the most abundant metal and the third most abundant element in the earth's crust,
after oxygen and silicon. It comprises about 8% by weight. Aluminum is too reactive chemically to
occur naturally as the free metal. Instead, it is found combined in over 270 different minerals. The
chief ore of aluminum is bauxite, a mixture of hydrated aluminum oxide (Al2O3·xH2O) and hydrated
iron oxide (Fe2O3·xH2O). Another mineral important in the production of aluminum metal is cryolite
(Na3AlF6). However, cryolite is not used as an ore; the aluminum is not extracted from it.
Metallic aluminum was first prepared by Hans Oersted, a Danish chemist, in 1825. He
obtained the metal by heating dry aluminum chloride with potassium metal.
AlCl3 + 3 K  Al + 3 KCl Al + 3 KCl
Robert Bunsen prepared aluminum metal in the 1850s by passing an electric current though molten
sodium aluminum chloride. However, because both potassium metal and electricity were quite
expensive, aluminum remained a laboratory chemical until after the invention of the mechanical
electrical generator. In 1886, Charles Martin Hall of Oberlin, Ohio, and Paul Héroult of France, who
were both 22 years old at the time, independently discovered and patented the process in which
aluminum oxide is dissolved in molten cryolite and decomposed electrolytically. The Hall-Héroult
process remains the only method by which aluminum metal is produced commercially.
The first step in the commercial production of aluminum is the separation of aluminum oxide
from the iron oxide in bauxite. This is accomplished by dissolving the aluminum oxide in a
concentrated sodium hydroxide solution. Aluminum ions form a soluble complex ion with hydroxide
ions, while iron ions do not.
Al2O3xH2O(s) + 2 OH¯(aq)  2 Al(OH)4¯(aq) + (x3) H2O(l) 2 Al(OH)4¯(aq) + (x3) H2O(l)
After the insoluble iron oxide is filtered from the solution, Al(OH)3 is precipitated from the solution
by adding acid to lower the pH to about 6. Then the precipitate is heated to produce dry Al2O3
(alumina).
| |
heat |
|
| 2 Al(OH)3(s) |
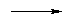 |
Al2O3(s) + 3 H2O(g) |
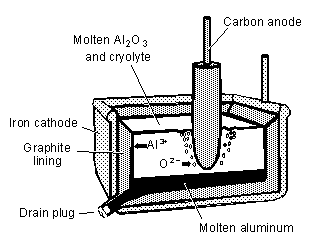
In the Hall-Héroult process, aluminum metal is obtained by electrolytic reduction of alumina.
Pure alumina melts at over 2000°C. To produce an electrolyte at lower temperature, alumina is
dissolved in molten cryolite at 1000°C. The electrolyte is placed in an iron vat lined with graphite.
The vat serves as the cathode. Carbon anodes are inserted into the electrolyte from the top. The
oxygen produced at the anodes reacts with them, forming carbon dioxide and carbon monoxide.
Therefore, the anodes are consumed and need to be replaced periodically. Molten aluminum metal is
produced at the cathode, and it sinks to the bottom of the vat. The principal cell reactions are
| cathode: |
4 Al3+ + 12 e¯ |
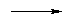 |
4 Al(l) |
| anode: |
6 O2¯ |
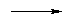 |
3 O2(g) + 12 e¯ |
| net: |
4 Al3+ |
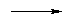 |
4 Al(l) + 3 O2(g) |
At intervals, a plug is removed from the vat and the molten aluminum is drained. The heat required
to keep the mixture molten is provided by resistive heating of the electrolyte by the current passing
through the cell. Typical cells use a potential of 4 to 5 volts and a current of 100,000 amperes.
Aluminum is a silvery-white metal with many valuable properties. It is light (density 2.70
g/cm3), non-toxic, and can be easily machined or cast. With an electrical conductivity 60% that of
copper and a much lower density, it is used extensively for electrical transmission lines. Pure
aluminum is soft and brittle, but can be strengthened by alloying with small amounts of copper,
magnesium, and silicon.
Based on its chemical reactivity, aluminum should not be very useful at all. Its standard
reduction potential is -1.66 volts, indicating that it is a very good reducing agent. It is far more active
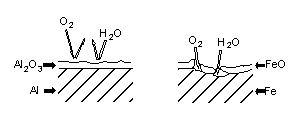
than iron, which has a reduction potential of -0.44 volt. Aluminum weathers far better than iron,
however, because the product of its corrosion, Al2O3, adheres strongly to the metal's surface,
protecting it from further reaction. This is quite different from the behavior of iron's corrosion
product, rust. Rust flakes off the surface of iron, exposing the surface to further corrosion.
The protective oxide coating on aluminum is frequently enhanced by the process of anodization.
Aluminum metal is made the anode in an electrolytic cell, where aluminum metal is oxidized and its
protective oxide coating is thickened. The oxide coating on aluminum renders it impervious to most
acids. However, any chemicals that form a strong complex with aluminum ions, e.g., OH¯ and Cl¯
ions, will react with the oxide coating. Therefore, aluminum will dissolve in hydrochloric acid and
sodium hydroxide solutions.
| Recycling of aluminum saves considerable energy. Because the aluminum is
already in the metallic state, all of the energy spent in purifying the ore and reducing it to
the metal is saved when aluminum is recycled. The aluminum needs only to be melted to
be reused. Aluminum has a rather low melting point, 660°C, and requires only 26 kJ/mol
to melt. To reduce a mole of Al from Al2O3 requires over 780 kJ. |
 |
EXERCISE: What major local company produces aluminum metal? Where do they get their electricity from (name)?
|